Design, Source, and Globally Test a Patient-Friendly Pharmaceutical Titration Pack (Rare Disease)Brand Management
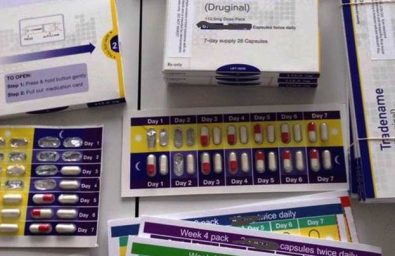
Design, test, and deliver a patient starter pack (titration pack) that meets the needs of a challenging patient population for a drug with dose form manufacturing limitations
Optio was hired to design drug packaging prototypes, manage the project, and conduct the patient market research to gather insights and confirm design changes.
Client need
The design of prototype packing and following patient market research must take into account:
- Patient population suffers from progressive decline in three symptom domains:
- Cognitive impairment
- Motor impairment
- Behavioral symptoms
- Titration packaging must incorporate four therapeutic dose levels
- Manufacturing limitations would not allow four unique dose forms (potential for patient confusion)
Market research methodology
Optio designed and sourced all primary research materials
- 4 week drug titration pack simulation (foam boards)
- 4 week drug titration pack concept image
- Pharmaceutical packaging prototype 1 and 2
- Paper + blister without box
- Optional text instructions
Optio conducted market research tests of pharmaceutical packaging designs with rare disease patients and caregivers
- Prototype 1 market research in the USA
- Prototype 2 market research in Italy and Germany
Results
- Optio successfully delivered final pharmaceutical package design recommendations to client
- Optio achieved a go-to-market final pharmaceutical titration package design. Client was satisfied that findings and insights were of such quality that further research was not required
- Client was prepared for an early potential launch of an important portfolio asset
